About Revlis
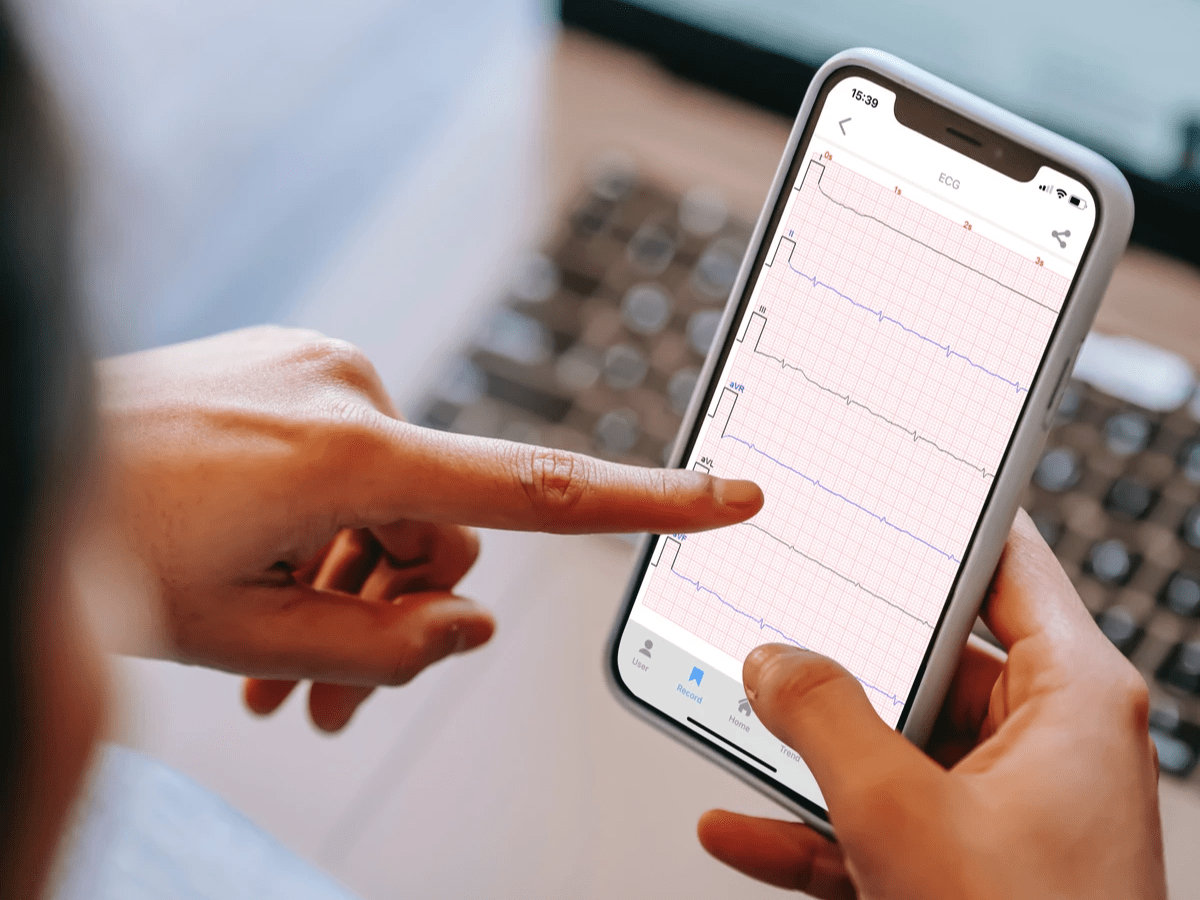
Since its establishment in 2003, Revlis Biotech Co., Ltd. has been committed to the development of digital health management. Through unique sensors and big data databases, it can measure a variety of physiological indexes originating from ECG, such as blood glucose, glycosylated hemoglobin, blood pressure, heart rate, etc. Among them, non-invasive blood glucose measurement is Revlis’ innovative and leading technology.
With the outbreak of the pandemic in 2020, global health and health awareness has rapidly increased, and people have greatly reduced the number of times they go to the hospital. As the market demand for home care gradually rises, Revlis actively promotes home self-monitoring solutions, so that we can instantly understand our physical condition wherever we are, and track your health trends for a long period of time to maintain good health.
Our Vision
Revlis is committed to developing health management solutions, serving the world, providing innovative and affordable solutions to manage and track personal health, and helping people enjoy a healthy life.
Our Service
As a provider of artificial intelligence technology, Revlis continues to develop more diversified physiological parameters and application levels in the field of ECG, and cooperates with technology industries and medical institutions in various fields to provide professional technical support, customized software development and big data operation and maintenance services, and provide innovative and affordable solutions to manage and track personal health and help people enjoy a healthy life.
Our Milestones

2023
Personalized ECG and physiological indicator data accumulation exceeds 5,000,000 records.
- ECG cumulative measurement seconds exceed 150,000,000.
- ECG cumulative measurement instances exceed 5,000,000.
- ECG cumulative symptom monitoring count exceeds 4,000,000.

2022
Cmate telecare system has achieved certification for the international medical information standard HL7 FHIR®
The Cmate telecare system has received international certification for medical information exchange standards. It is integrated with the global digital medical field, serving as a provider of secure cloud-based medical solutions in the industry.

2021
Awarded the international GHP Best Non-Invasive Medical Technology Company 2021

2020
- Development of a Non-Invasive Blood Sugar AI Model
- Construction of a Relational Database for ECG and Physiological Indicators, along with a Central Data Management System
The launch of AI-Glu Smart ECG Cloud Computing Module, promoting modular ECG computing solutions.

2019
Establishing a global strategic partnership with Hatari group (Thailand) to build a large health market
The second-generation sensor "Xin Meite" G2-SE Heartbeat Recorder has obtained a Thai medical device license. This partnership also includes collaboration with 10 major local hospitals in Thailand to establish a service ecosystem for medical and health care.

2018
- Launch of the second-generation sensor "Xin Meite" G2-SE Heartbeat Recorder
- Revlis received the Intel IoT Smart Healthcare Personal Health Market Ready Solution
Revlis was invited to the annual Intel Retail Solution Day, where, with its AI intelligent medical solutions and extensive experience in localized intelligent health and medical services in China, it became an Intel MRS (Market Ready Solution) partner.

2015
Revlis has formed a global strategic partnership in telecare with Tunstall (UK)
Utilizing its advanced sensor devices and remote medical collaboration services, Revlis has partnered with the Tunstall Group, one of the top three global telehealthcare institutions, to establish a remote technology medical service business in Mainland China. This initiative was later selected as a key project for the UK Health Minister's visit to China to observe telehealth services.

2013~2016
Cmate 1st portable ECG device has obtained medical device licenses both domestically and internationally
- In 2013, it received the Taiwan TFDA (Taiwan Food and Drug Administration) medical device license.
- In 2014, it obtained the CFDA (China Food and Drug Administration) medical device registration certificate in Mainland China.
- In 2015, it was registered with the Australian TGA (Therapeutic Goods Administration) for therapeutic products.
- In 2016, it achieved the European Union's CE certification for medical devices.
Contact us
No.108-1, Minquan Rd., Xindian Dist., New Taipei City 231, Taiwan (R.O.C.)+886-2-22198878
© 2003 - 2023 Revlis